[

Greater than three dozen hand sanitizers and aloe gel are reportedly being recalled as a result of they include a extremely poisonous substance that may trigger severe well being issues together with blindness and coma.
The FDA stated Friday that Aruba Aloe Balm NV, based mostly in Oranjestad, Aruba, is “voluntarily recalling” 40 plenty of Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholda Gel. These merchandise include alcohol modified from methanol.
Because the FDA notes, publicity to methanol could cause nausea, vomiting, headache, blurred imaginative and prescient, coma, seizures, everlasting blindness, everlasting injury to the central nervous system, or loss of life. The folks most in danger for methanol poisoning are youngsters who by chance swallow these merchandise and youths and adults who drink these merchandise as an alternative choice to alcohol (ethanol).
Aruba Aloe Balm NV claims that it has not acquired any studies of opposed occasions related to using its merchandise.
Aruba Aloe Hand Sanitizer Gel is used as a sanitizer to assist cut back micro organism that may probably trigger sickness. It’s packaged in 12 fl ounce inexperienced plastic bottles with a white label studying, “Aruba Aloe Hand Sanitizer Gel 80% Alcohol, Made in Aruba with the World's Best Aloe.”
Aruba Aloe Alcoholda Gel is designed to offer momentary reduction from ache and itching related to minor burns, sunburns, insect bites or minor pores and skin irritations. It is available in 2.2 and eight.5 fl. Ounce Clear Bottles.
The next are the affected Aruba Aloe Hand Sanitizer GEL product heaps:

The affected Aruba Aloe Alcoholda Gel product heaps are as follows:
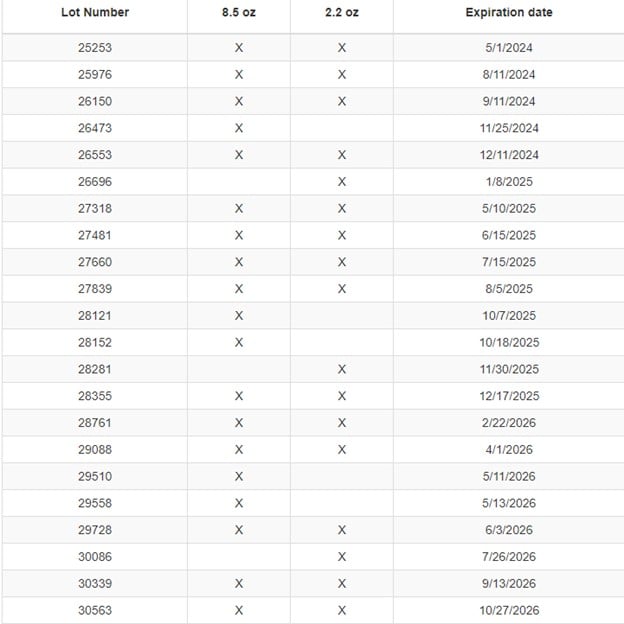
Based on the FDA, the merchandise had been distributed between 5/1/2021 and 10/27/2023 and bought on-line within the US by the Aruba Aloe Balm NV web site. The Aruba-based firm is notifying prospects who’ve bought merchandise through e mail and providing low cost coupons for future purchases.
The FDA is urging customers to cease utilizing the merchandise and throw them away. The company additionally says prospects affected by the product ought to contact their physician or well being care supplier.

